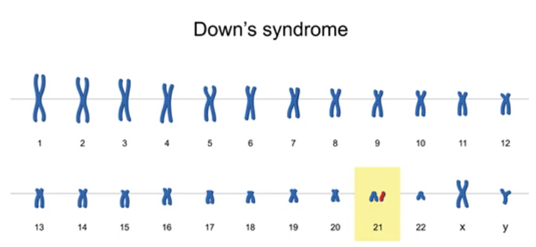
 In 1866, Dr. John Langdon Down described and published a complete description of the typical signs of Down syndrome for the first time. Therefore, the syndrome was named Down syndrome after his name. It was confirmed in 1959 that Down syndrome was caused by chromosomal abnormalities. Modern medicine has confirmed that the incidence of Down's syndrome is related to the mother's age at pregnancy, that is, the abnormality of chromosome 21, which has three types: trisomy, translocation, and mosaicism. Older pregnant women and the aging of the ovum are important causes for non-separation. The older the mother, the higher the risk of the disease. Pregnant women over 40 years of age undergo amniocentesis screening, the detection rate of the disease is more than 5%. 60% of the children are aborted in the early stages of the fetus, and the survivors have obvious mental retardation, special facial features, growth and development disorders, and multiple deformities.
In 1866, Dr. John Langdon Down described and published a complete description of the typical signs of Down syndrome for the first time. Therefore, the syndrome was named Down syndrome after his name. It was confirmed in 1959 that Down syndrome was caused by chromosomal abnormalities. Modern medicine has confirmed that the incidence of Down's syndrome is related to the mother's age at pregnancy, that is, the abnormality of chromosome 21, which has three types: trisomy, translocation, and mosaicism. Older pregnant women and the aging of the ovum are important causes for non-separation. The older the mother, the higher the risk of the disease. Pregnant women over 40 years of age undergo amniocentesis screening, the detection rate of the disease is more than 5%. 60% of the children are aborted in the early stages of the fetus, and the survivors have obvious mental retardation, special facial features, growth and development disorders, and multiple deformities.
There are several ways to test Down syndrome:
Cytogenetic test. The karyotype analysis of peripheral blood cell chromosomes can diagnose and type Down syndrome. According to the chromosomal karyotype, children with the disease can be divided into standard type, translocation type, and mosaic type.
Fluorescence in situ hybridization. Using the corresponding fragment sequence of chromosome 21 as a probe, hybridizing with lymphocytes or amniotic fluid cells in peripheral blood can accurately locate the abnormal part of chromosome 21.
Serum marker screening. Down syndrome screening is performed during the first or second trimester of pregnancy by measuring serum chorionic gonadotropin (β-HCG), alpha-fetoprotein (AFP), and free estriol (FE3).
Ultrasonic detection. The thickness of the transparent layer of the fetal neck is also an important screening index for Down syndrome. The higher the value, the greater the risk.
Chromosome examination of amniotic fluid cells. At present, the most commonly used technique is amniocentesis, an ultrasound-guided extraction of amniotic fluid for chromosomal karyotype analysis of fetal cells.
Creative Biogene's test kits are mainly used to determine relevant markers in human serum or amniotic fluid in vitro through serum marker screening and other methods, so as to assist in the diagnosis of Down syndrome. Our products are simple and convenient to operate, can quickly obtain accurate detection results, and have high sensitivity and specificity. It is the best choice for your related research.
Creative Biogene's skilled scientists have extensive experience in developing diagnostic products for Down syndrome. It is sincerely looking forward to cooperating with you and providing you with the best quality product with all our hearts! You can choose us without hesitation.
Please contact us for more details.
References
| Cat# | Product Name | Product Type | Inquiry |
|---|---|---|---|
| C1300T | AFP IRMA test kit | Test kit | Inquiry |
| C1301T | beta-hCG free ELISA test | Test kit | Inquiry |
| C1302T | PAPP-A ELISA test | Test kit | Inquiry |
| C1303T | beta-hCG free IRMA test | Test kit | Inquiry |
Copyright © 2026 Creative Biogene. All rights reserved.